
Haemophilia A - for which there is no known cure – has inflamed Indian researchers to the world stage as they pioneer the first ever human gene therapy to halt the hereditary bleeding disorder. The first such treatment, pioneered by the Centre for Stem Cell Research (CSCR), Christian Medical College (CMC), Vellore, under the Department of Biotechnology of the Indian government has brought positive results in first phase clinical trial.
The Breakthrough
The gene therapy involves a lentiviral vector delivering a Factor VIII gene into patient-specific autologous HSCs. These modified HSCs subsequently produce blood cells that release functional Factor VIII for significantly longer intervals. This innovative approach effectively addresses the problem of resource-demanding Factor VIII replacement therapy used in treatment of Haemophilia A but posing multiple challenges including high costs, problems with access to veins, and patient compliance.
Clinical Trial Results
The clinical trial in this study involved five subjects aged between 22 and 41 and shown transformative outcome. The five included patients had no bleeding episodes in one year, providing a follow up time of 81 months in aggregate. This means that for the over one-year follow up period none of the participants experienced any bleeding event, something that is not achieved when using the current standard treatment.
Implications of Patients with Haemophilia
According to the findings of the research, the prevalence of haemophilia A in India is roughly 1.3 Lakh persons, making it the country with the second-largest population of haemophilia A in the whole globe. The current treatment requires regular infusions of Factor VIII, which can be both inconvenient and costly. The new gene therapy is promising for the treatment of the disease in that it touches on the source of the problem with an additional likelihood of long-term production of Factor VIII.
Advantages of Gene Therapy
Gene therapy can be therefore concluding from this view as another world of treatment of genetic disorders. It is a one-time treatment which is introduced in the form of a functional gene into the cells of the patient that have the abilities to yield long term effects. This is especially beneficial for patients in developing states, where access to regular infusions and medical care is often limited or impossible.
Future Prospects
This trial achieved the much needed successful attempt in the treatment of Haemophilia A though gene therapy. During the subsequent stage of the clinical study, a bigger group of volunteers will be recruited in order to conduct further assessments of the treatment's effectiveness and safety. If similar results are achieved, this therapy may soon be put to practice and may serve as a helpful therapy for thousands of patients with this disabling disorder.
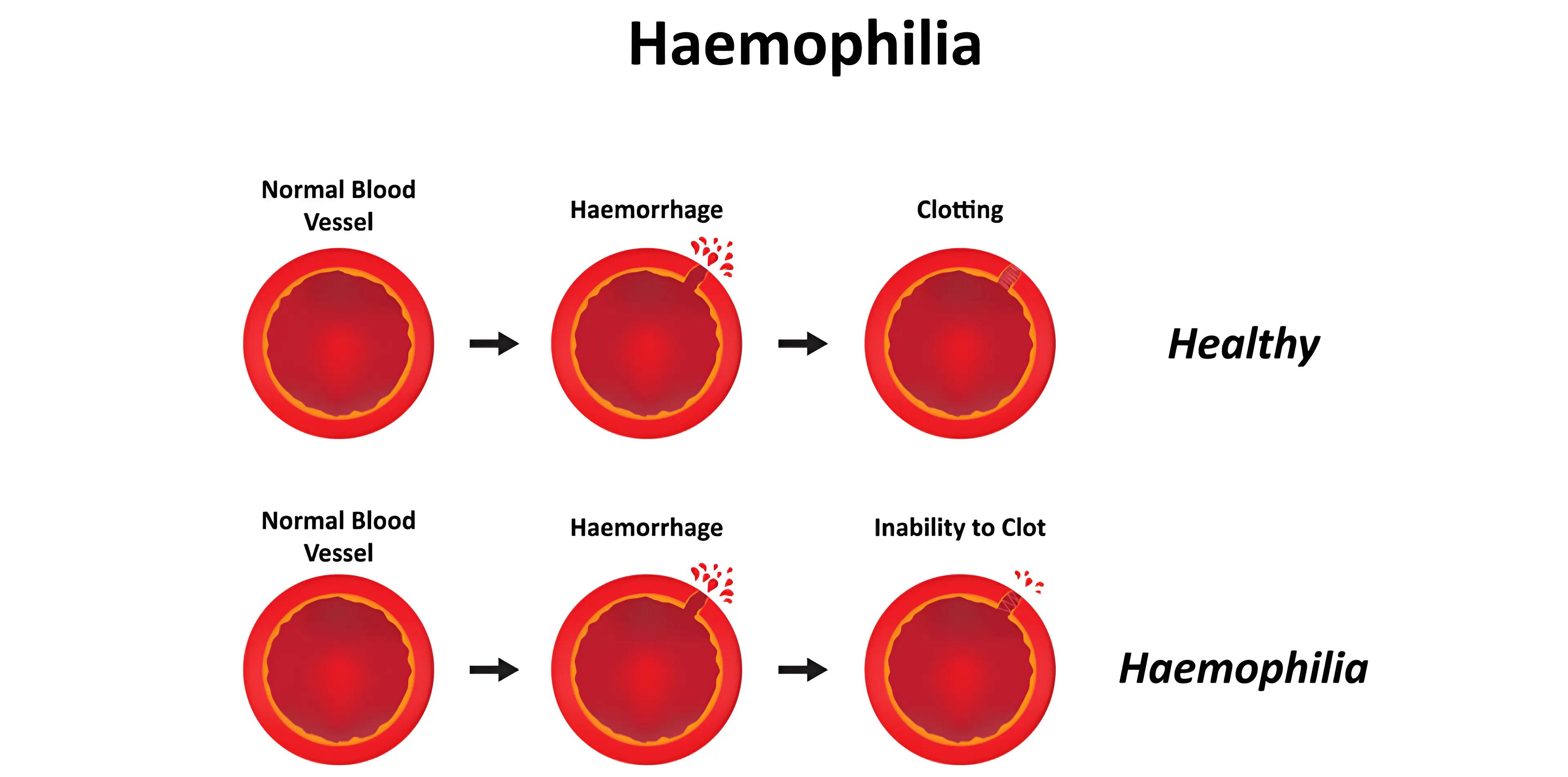
Understanding Haemophilia A
Haemophilia A is a rare bleeding disorder caused by a deficiency of coagulating Factor VIII in blood. This condition is found mainly in males because the gene through which the disease is inherited is present in the X chromosome but can also affect females with a defective gene. Consequently, Haemophilia A patients take longer to clot than normal people, and they can have life threatening bleeding episodes even with injury.
Genetic Basis
Haemophilia A belongs to the group of hereditary diseases inherited through the X chromosome, specifically it is X linked recessive. Male individuals are affected if they possess one normal X chromosome and one Y chromosome and the X chromosome has the defective gene. Females, with two X chr. are usually carriers if one of the chr. has the mutation, while the other chr. can balance for the defect. Once in a while female carriers do show symptoms especially if both the X chromosomes are damaged.
Symptoms and Diagnosis
The severity of Haemophilia A varies depending on the level of Factor VIII in the blood:
- Severe Haemophilia: Defined by Factor VIII coagulant activity of less than 1% of normal activity that produces severe spontaneous bleeding events multiple times a mainly in the joints and muscles.
- Moderate Haemophilia: A condition characterized by Factor VIII levels of between 1-5 % with bleeding initiated following injury or surgery.
- Mild Haemophilia: According to Factor VIII levels that range from 5-40 %, where bleeding is rare and usually occurs only after significant trauma or surgical procedures.
Common symptoms include:
- Spontaneous Bleeding: Especially when it occurs in the joints, which results in pain, joint swelling and long-term joint dysfunction known as hemarthrosis.
- Prolonged Bleeding: After injuries, surgeries or any dental work.
- Unusual Bleeding Patterns: For example, when a female with mild Haemophilia experiences sometimes recurrent nose bleeds or abnormally severe menstrual flow.
The diagnose for Haemophilia A includes a series of tests on clotting factors found in the body. The specific mutation in Factor VIII gene can be detected based on the genetic testing for determining the carriers and prenatal analysis.
Treatment and Management
The major management of Haemophilia A is substitution therapy, involving the administration of recombinant Factor VIII concentrate. It can be done as a burst activity (for managing bleeding events) or as scheduled (for preventing bleeds). New treatments that were developed have recombinant Factor VIII products thereby lowering the probability of individual contracting diseases from blood products.
Even the novel methods of treating the gene that causes the disorder indicated by recent victories in gene therapy suggest long-term aims at dealing with the problem of the disorder. Although gene therapy has not yet been directly compared to other therapies in clinical trials, earlier trials of gene therapy have revealed that the functional copy of the Factor VIII gene introduced into the patient’s cells helps decrease bleeding episodes and the frequency of the injections.
Challenges in Treatment
Despite advancements, several challenges remain:
- Inhibitor Development: Some patients have the ability to form molecules called inhibitors which reduce the effectiveness of infused Factor VIII.
- Access and Cost: Costs of Factor VIII concentrates and gene therapy are relatively high and can therefore be prohibitive especially in developing countries.
- Adherence: This has its drawbacks since the regular injecting cause’s burden to patient’s hence poor compliance mostly among the children and adolescent.
Living with Haemophilia A
Children and adults diagnosed with Haemophilia A can live generally healthy lives if their condition is well managed and receives appropriate medical treatment. Avoid activities that involve contact, wear protective equipment when possible, to decrease chances of a bleed. Check-ups and physiotherapy sessions are key to reducing some joints pains and mobility.
Support from healthcare providers, patient organizations, and communities are crucial. Practical measure can help educate the general public, and demystification of with a view to enhance early diagnosis and interventions. In families with haemophilia A, genetic counsellors offer useful information on paternity, marriage and reproductive options.
Stem Cell Research: Future Prospective of Regenerative Medicine
Stem cell research is a relatively young scientific field that entails constant growth and seems to be closely connected with the future of regenerative medicine, disease treatment, and more. Stem cells are special due to their capacity to divide and produce more stem cells and more specialized cells. Due to this versatility stem cells have become the center of focus in the analysis of curing as many diseases and injuries as possible. Here is sharp overview of stem cells and the hope for future advancement.
Types of Stem Cells
Stem cells can be broadly categorized into several types based on their origin and potential:
- Embryonic Stem Cells (ESCs): Derived from early stage embryos, ESCs are referred to as being pluripotent or they have the ability to turn into any cell in the body. They can be used very effectively in research on prenatal human development and likely treatments for many diseases. However using embryos has created a number of ethical issues that subject to debate and close scrutiny of regulators.
- Adult Stem Cells: Adult stem cells are also referred to as somatic, or tissue particular stem cells these are present in different tissues of the body including bone marrow, skin, and the brain. These cells described as multi-potent means that stem cells are only able to transform into a finite number of required cells associated with the tissue in which the stem cells are found. A stem cell is an adult in the sense that it does not generate all the tissues of the adult body but is involved in other healing activities on the body such as bone marrow transplants.
- Induced Pluripotent Stem Cells (iPSCs): iPSCs are adult cells that have been genetically reprogrammed to a pluripotent state like ESC. This process initiated by Shinya Yamanaka can be used to produce stem cell that is unique to the patient’s condition without the issues that comes with embryonic stem cells research. iPSCs have potential uses in personalized medicine, drug and toxicity testing, and disease modelling.
Uses & Benefits of Stem Cell Research
The potential applications of stem cell research are vast and transformative:
- Regenerative Medicine: Stem cell treatment is a process that seeks to replace vital body structures that have been impaired through illness. For instance, stem cell therapy may be applied for repairing the damaged cardiac muscle being insufficient after a heart attack, producing insulin for diabetes patients or generating nerve cells to treat spinal cord injuries and other neurodegenerative diseases including Parkinson’s and Alzheimer’s.
- Disease Modelling and Drug Testing: Besides, iPSCs can produce genetically modified cell lines which can be used to model the diseases in a dish in lab. This allows researchers a view into disease processes at the cellular level and evaluate and new drugs for therapeutic value. Eventually it will lead to enhanced treatment and can decrease the utilization of animal testing.
- Understanding Human Development: It is possible to gain important knowledge of human ontogenesis and of the mechanism of cell differentiation and tissue formation studying stem cells. This can enable the scientists understand causes of congenital abnormalities and the foundations of various diseases.
Challenges & Ethical Perspective
While the potential of stem cell research is immense, several challenges and ethical considerations must be addressed:
- Ethical Concerns: In the course of embryonic stem cell research, a moral issue arises through deletion of the embryos. This as a result has made many regulations and restrictions in many different countries. When using iPSCs there is a means that was developed to sidestep some of the issues, however, a continuing discussion and ethical analysis is necessary.
- Technical Challenges: It becomes necessary to safeguard patients from stem cell treatments and look into its efficiency. This include; the ability to control differentiation process and the formation of tumour and immunity against the transplant. Advances in gene editing technologies, such as CRISPR-Cas9, are helping to address some of these issues.
- Regulatory Hurdles: Bringing stem cell therapies from the lab to the clinic involves navigating complex regulatory pathways. As a result, there is a great scrutiny and control in an effort to evaluate the treatment and ensure it to be approved to be safe and efficient.
Future Prospects
Stem cell research is one of the most explosive sciences at the present time, so it is rather promising. Fundamentals of gene editing and tissue engineering, progress in the molecular biology toward personalized medicine dealing with number of disease contingencies are in progress. The result of subsequent work should be focused on the scientists, clinicians, regulatory authorities, and ethicists in order to extend the results of such studies to enhance the quality of peoples’ lives and health.