Dr Bhanu’s research group from Institute of Nano Science and
Technology (INST), Mohali, a deemed autonomous institute of
Department of Science &Technology has developed new method
to clean Cr(VI) ions with the help of sunlight for the catalytic
process and combining the micro fluidic technique to convert
hexavalent chromium to less hazardous trivalent in presence
minimum concentration of chemical reagents. They employed
a technique called continuous flow photo-reduction and
verified this process in wastewater using TiO2 nanoparticles.
Besides, the cost effectiveness of the process and the
usage of renewable energy with the microfluidics route,
the reduction efficiency can be also controlled by fine-tuning
the flow rate of the organic pollutant, reactor dimension,
and architecture, perceptually. Another advantage of using
micro reactors is that photo catalyst could be reused without
needing any recovery agents or complicated procedures.A
cheap solution has been invented by INST researchers to
filter out toxic Chromium from wastewater used in leather
tanning and electroplating industries; this uses sunlight
as a catalyst and also employs microfluidic technology.
Hexavalent chromium is very dangerous and according to
the WHO reports the permissible level of hexavalent and
trivalent chromium in drinking water is zero.
These concentrations were found to be 05 mg/L and 5 mg/L.
Hence, it is necessary to go ahead and decrease hexavalent
of this chromium to trivalent chromium. Some of the
chemical and physiochemical treatments that have been
used in the removal of Cr(VI) include; ion exchange
treatments, adsorption, and bacterial as well as
chemical reductions. Many of these techniques are
rather expensive and the removal efficiencies of Cr(VI)
are rather low.
Current ways by which toxic Chromium is removed from the waste water of industries:
- Chemical Precipitation:This method involves the use of chemical precipitation whereby chemicals are used from insoluble compounds with chromium ions. These compounds then get precipated out in a solid form and which can be filtered and removed.
- Ion Exchange: in the process the wastewaters less electrically charged by giving away unwanted ions in the wastewater for other ions bound to a solid phase.Ion exchange resins are used to selectively attach onto chromium ions, instead exchange in the way it becomes something that is a lot less lethal.
- Coagulation and Flocculation: a process in which tiny flocks in the wastewater come together to form bigger entities, known as flocs.The flocs or aggregates are persuaded to grow larger by gentle stirring or mixing.In the end they become flocs which settle down at the bottom of the vessel with chromium.
- Membrane Filtration: it allows water molecules to move free and pass through the membrane but doesn’t allow the chromium ions to pass through the membrane.Reverse osmosis and ultrafiltration belongs to the most widely used type of membrane filtration processes.
Sunlight-Powered Chromium Removal Technique

The Problem:area of interest here is chromium in the industrial wastewater. Out of all the chromium, it is the hexavalent chromium; Cr(VI) that poses high risk of health and environmental impact.Some industries such as leather tanning and electroplating for instance, are well known to release high amounts of chromium into water.
The Breakthrough: Dr. BhanuPrakash along with his team at INST has innovated an effective and cost efficient solution.They use two powerful allies: sunlight and micro fluidics innovation technology.
How It Works: Picture a small sized reactor with sunlight writhing with nanoparticles. Here’s the step-by-step:
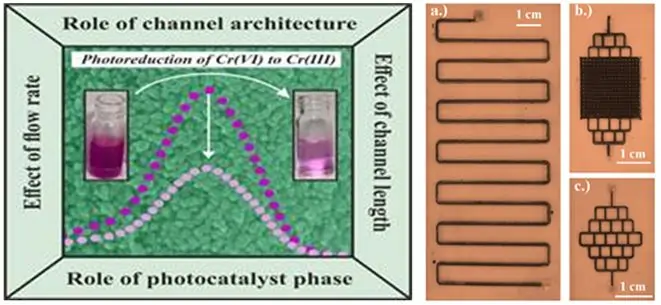
Optical image of the micro reactors fabricated using laser micromachining technique with
- Serpentine (μR-S).
- Branched (μR-B).
- Micro pillars (μR-M) based architectures.
- Continuous flow photo-reduction: The researchers use TiO₂ nanoparticles (photo-catalyst) in a microfluidic reactor.This reactor is where the wastewater goes through; on reaching this section, sunlight stimulates the TiO₂ nanoparticles.
- Hexavalent to Trivalent Transformation: Hexavalent chromium (Cr(VI)) is the culprit here and need to be neutralised.When exposed to sunlight, the TiO₂ nanoparticle starts working.It converts Cr(VI) to the less toxic Cr (III).Cr(VI) is very toxic and can cause diseases in the human body.
- Precise Control with Microfluidics: Microfluidic technology ensures that parameters such as flow rate as well as the size of the reactor can be well managed.This increases the reduction efficiency – similar to a conductor sharpening the group of musicians.
What Is Hexavalent Chromium?
Chromium (Six) or the state +6 oxidation state chromium is
a chemical compound of Chromium.It is present in various
ions which include chromate ion (CrO₄²⁻) and dichromate
ion (Cr₂O₇²⁻).
Occurrence and Uses:
- Hexavalent chromium is a rather scarce in nature, while in industries; it is produced in big amounts.
- It finds applications in:
- Chromate pigments: It is used in the manufacture of dyes, paints and plastics among others.
- Anticorrosive coatings: Along with paints and primers, it is used as anticorrosive agent.
- Electroplating: Offers protection to different metallic products.

Health Risks:
- Chromium in the hexavalent form is acknowledged to be carcinogenic to humans and its inhalation is included in the IARC Group 1.
- Those employees working (for instance welders, chromate handlers) in factories inhales are vulnerable to many health issues as lung cancer, asthma, skin problems among others.
Formation: at high temperatures like welding turn chromium into its hexavalent state.
Safety Measures: Hexavalent chromium should always be avoided to the extent possible so that health of people is not affected.
Difference between Trivalent Chromium and Hexavalent chromium
A. Trivalent Chromium (Cr(III)):
Stability and Occurrence:
- It is highly stable, and exists in the earths’ crust combined with specific minerals.
- This element is in +3 oxidation state.
- Water insoluble.
Low Toxicity:
- Cr(III) is less toxic as compared to its counterpart.
- It is also considered as part of human nutrition.
Functions:
- Cr(III) has an important part in the regulation of glucose and insulin.
B. Hexavalent Chromium (Cr(VI)):
Solubility and Colour:
- It easily gets dissolved in the water and is highly soluble.
- it is yellowish in colour.
Toxicity and Hazards:
- Cr(VI) is toxic to human beings and has some technical health hazards.
- It is highly carcinogenic in nature and can causes lung cancer.
- They are closely monitored by the EPA.
Industrial Use:
- Present in usage in industrial systems such as electroplating.
- Exogenous – most definitely not part of our dinner plate of choice foods.
Institute of Nano Science and Technology (INST), Mohali
- Mission and Establishment: INST was founded under the NANO MISSION which was started by the Department of Science and Technology (DST), Government of India. Nano science and Nanotechnology research and development is what it is majorly involved in.
- Autonomous Institution: INST being an autonomous research institute functions under the provision of the Society Registration Act, 1960. There is this autonomy in the determination of its research focuses and work plan, it vests in¬.
- Research Areas: INST covers a wide spectrum of research programs, including: Physical Sciences, Chemical and Biological Sciences and Interdisciplinary Sciences.
- It addresses critical issues related to: Energy,Environment, Quantum Materials,nano-devices,chemical Biology
- Sunlight-Powered Chromium Removal Technique: More significantly, the INST has developed a new approach to the elimination of chromium from the wastewater using sunlight. This discovery has important implications for sustainable existence of the environment.